Knowledge Base Category -

Did You Know?
Breast Cancer Awareness Month was first celebrated in October 1985 as partnership between the American Cancer Society and Imperial Chemical Industries (now AstraZeneca).
Chances are you; a family member, close friend or acquaintance has been impacted by breast cancer. October is Breast Cancer Awareness Month. According to the CDC, each year:
- About 240,000 women in the United States get breast cancer and 42,000 women die from the disease,
- Men can also get breast cancer, but it is not common. About one out of every one hundred breast cancers diagnoses in the United States is found in a man, and
- While most breast cancers are found in women who are 50 years old or older, breast cancer also affects younger women.
Why Should You Care?
Even though family history increases the risk of breast cancer, most women diagnosed with breast cancer have no known family history of the disease. Early detection allows for a higher chance of cure. Mammography is used to detect breast cancer and is one of many Preventative Services covered by Medicare.
What Can I Do?
Know Ways to Lower Your Risk for Breast Cancer
The CDC details thing you can do to help lower your risk of breast cancer including:
- Keep a health weight and exercise regularly,
- Choose not to drink alcohol, or drink alcohol in moderation,
- If you are taking hormone replacement therapy or birth control pills, ask your doctor about the risks, and
- Breastfeed your children, if possible.
Know the Warning Signs of Breast Cancer
While there are different symptoms of breast cancer, and some people have no symptoms at all, symptoms can include:
- Any change in the size or shape of the breast,
- Pain in any area of the breast,
- Nipple discharge other than breast mild (including blood),
- A new lump in the breast or underarm, thickening or swelling or part of the breast,
- Irritation or dimpling of the breast,
- Redness or flaky skin in the nipple area of the breast.
Be Your Own Patient Advocate
If you have any signs or symptoms that worry you, follow-up with a health care provider as soon as possible.
Talk to your health care provider about when and how often to get a screening mammogram. If you are worried about the cost, the CDC’s National Breast Cancer Early Detection Program (NBCCEDP) (https://www.cdc.gov/cancer/nbccedp/screenings.htm) provides breast and cervical cancer screenings and diagnostic services to women who have low incomes and are uninsured or underinsured.
Beth Cobb
September is Prostate Cancer Awareness Month. Prostate cancer is the second leading cause of male cancer-related death in the U.S.[1] According to the American Cancer Society, it is estimated that in 2023 there will be 288,300 new cases of prostate cancer and 34,700 prostate-cancer related deaths in the U.S.[2]
Historically, there have been limited options in managing patients with advanced prostate cancer. However, in the last several years, we have seen remarkable progress in the development of new diagnostic and therapeutic tools. One of these, PSMA PET imaging for prostate cancer, is a particularly exciting development and is the focus of this article. Medical oncologist Michael Morris from Memorial Sloan Kettering Cancer Center calls this new imaging technology “the biggest advance in prostate cancer detection since the PSA test was developed in the 1980s.”[3]
PSMA PET Imaging: A New Diagnostic Tool
Prostate-Specific Membrane Antigen, or PSMA, is a protein that is present at a higher level in prostate cancer cells, and in addition, is often found on the surface of prostate cells.[4] These characteristics of PSMA make it a good target for imaging prostate cancer that might have escaped from the prostate and traveled to other parts of the body. PSMA should not be confused with Prostate-Specific Antigen, or PSA, which is a protein produced by the prostate.[5] The PSA test measures the level of PSA in the blood. An elevated PSA in the blood can be an indication of prostate cancer, although it can be due to other factors.
Imaging for advanced prostate cancer has been problematic for many years, with men often having to undergo a conventional CT scan and a bone scan to see if there is evidence of metastatic disease. However, according to the National Cancer Institute, both of these conventional imaging technologies have limitations since “neither is particularly good at finding individual prostate cancer cells, and thus can miss very small tumors.”[6] PSMA PET imaging promises to improve the sensitivity of detecting prostate cancer metastases compared to conventional imaging approaches, and thereby better inform the treatment and management of patients with advanced disease.[7]
Clinical trials have shown some promising results for this new imaging technology. For example:
- In the CONDOR trial, a total of 208 men were enrolled in the study. The men had a rising PSA after surgery or radiotherapy. The study evaluated the radiotracer 18F-DCFPyL and its ability to detect prostate cancer in these men when performing a PET/CT. The trial demonstrated that the radiotracer correctly localized disease in approximately 85% of men with prostate cancer biochemical recurrence, all of whom had uninformative conventional imaging.[8]
- In another trial, 276 prostate cancer patients were enrolled to evaluate the clinical impact of 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography on the planned management of prostate cancer patients with biochemical recurrence after surgery.[9] It was found that the use of this imaging technology allowed clinicians to radically change the intended treatment approach before imaging evaluation, in roughly two out three individuals.
FDA Approvals
During the last several years, the FDA has approved several radioactive tracers for use in PSMA PET imaging. For example:
- On December 1, 2020, the FDA approved the radioactive tracer Gallium (Ga) 68 PSMA-11 for use in PET imaging of patients with suspected prostate cancer metastasis who are potentially curable by surgery or radiation therapy.[10] The tracer can also be used for patients with suspected prostate cancer recurrence based on elevated serum PSA levels.
- On May 26, 2021, the FDA approved a second PSMA-targeted PET imaging drug, Pylarify (piflufolastat F 18), for the same prostate cancer imaging indications as Ga 68 PSMA-11.[11] The FDA noted that with this approval, certain men with prostate cancer will have greater access to PSMA-targeted PET imaging that can aid health care providers in assessing prostate cancer.
Additional FDA approvals have followed for Illuccix (gallium Ga 68 gozetotide) (12/17/2021)[12], Locametz (gallium Ga 68 gozetotide) (3/23/2022)[13], and Posluma (flotufolastat F 18) (5/25/2023)[14].
Once a PSMA-targeted radioactive tracer is injected into the patient, the tracer travels throughout the body and attaches to PSMA; the cells thus flagged will then “light up” when a PET scan is performed.[15]
PSMA Tracers by the Numbers
To measure the growth in utilization of this new imaging technology, RealTime Medicare Data constructed a Tableau visualization using its nationwide Medicare Fee-for-Service (FFS) paid claims database. Here is some key trending information from that visualization:
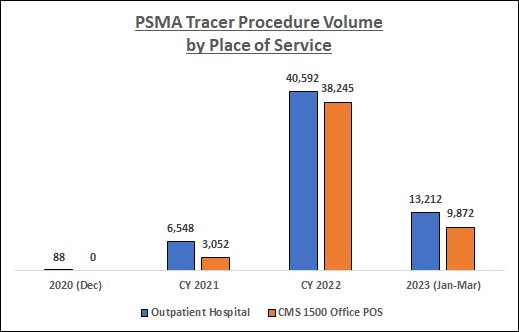
Data Source: RealTime Medicare Data, LLC. Time period: 12/1/2020-3/31/2023. Geography: all 50 states and D.C. CMS 1500 Office POS and Outpatient Hospital Medicare Fee-for-Service. The following HCPCS Codes were included in the data queries: A9593-GALLIUM GA-68 PSMA-11 DIAGNOSTIC UCSF 1 MCI, A9594-GALLIUM GA-68 PSMA-11 DIAGNOSTIC UCLA 1 MCI, A9595-PIFLUFOLASTAT F-18 DIAGNOSTIC 1 MCI, A9596-GALLIUM GA-68 GOZETOTIDE DIAG ILLUCCIX 1 MCI, A9597-POSITRON EMISSION TOMOGRAPHY RP DX TUMOR ID NOC, and A9800-GALLIUM GA-68 GOZETOTIDE DIAGNOSTIC 1 MCI. PDx's not related to prostate cancer, and CPT Modifier 26 (relating to professional fees), were filtered from the data.
As the above chart indicates, PSMA tracer utilization among the Medicare FFS population has increased substantially over the study period, especially during CY 2022. In addition, there has been a shift in the place of service where these procedures are being performed; indeed, by 2022 procedure volume in the Office setting was fast approaching that in the Outpatient Hospital setting.
From Imaging to Targeted Therapy
In addition to being a target for prostate cancer imaging, can PSMA be a target for prostate cancer therapy? In 2022, the FDA weighed into this question with these exciting developments:
- On March 23, 2022, the FDA approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with PSMA-positive metastatic castration-resistant prostate cancer who have been treated with androgen receptor pathway inhibition and taxane-based chemotherapy.[16]
- On the same day, the FDA approved the radioactive tracer Locametz (gallium Ga 68 gozetotide) for positron emission tomography (PET) of PSMA-positive lesions, including the selection of patients with metastatic prostate cancer for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated.[17] The FDA noted that Locametz is the first radioactive tracer approved for patient selection in the use of a radioligand therapeutic agent.
Pluvicto acts by binding to PSMA; a radioactive particle then kills the cancer cells.[18] In a clinical trial leading to the FDA approval, the trial demonstrated a statistically significant improvement in the primary endpoints of overall survival and radiographic progression-free survival.[19]
Questions remain as to who might be able to receive the new therapy drug beyond those who have already been treated with chemotherapy, whether it will benefit patients during earlier stages of prostate cancer, and whether its effectiveness will be improved if combined with other therapies.[20]
Disclaimer: This article does not provide medical advice. It is intended for general informational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in this article.
Curtis Spraitzar
Did You Know?
August is National Immunization Awareness Month (NIAM). According to the CDC, NIAM “is an annual observance held in August to highlight the importance of routine vaccination for people of all ages.”
Why It Matters?
Immunity from childhood vaccines can wear off over time. Keeping your vaccinations up to date throughout life helps you combat vaccine preventable diseases. The CDC advises that all adults need a COVID-19 vaccine, Influenza (flu) vaccine every year, and Tetanus and diphtheria (Td) or Tetanus, diphtheria, and pertussis (Tdap) vaccine every ten years.
Additional Key Vaccines
Shingles Vaccine (Shingrix)
The two-dose series of shots to protect against shingles. The CDC cites that “in adults 50 years and older who have healthy immune systems, Shingrix is more than 90% effective at preventing shingles” and postherpetic neuralgia (PHN)< the most common complication from shingles.”
Pneumonia Vaccines
In 2022, the percent of adults aged eighteen and over who had ever received a pneumococcal vaccination was only 23.9%. Older adults are at greatest risk of serious illness and death from pneumococcal disease. In the United States, there are two kinds of vaccines to help prevent pneumococcal disease, Pneumococcal conjugate vaccines (PCV 13, PCV15, and PCV20), and Pneumococcal polysaccharide vaccine (PPSV23).
PCV13: Prevnar 13® (pneumococcal conjugate vaccine or PCV13) is a registered trademark by Wyeth LLC and marketed by Pfizer Inc. This vaccine provides protection against infections caused by six more serotypes than PCV7. This vaccine is part of the routine childhood immunization schedule. Additionally, in 2011, it was licensed by the FDA for use in adults 50 years or older.
The CDC recommends PCV13 for all children younger than 2 years old, and people 2 years or older with certain medical conditions.
The CDC advises adults 65 years and older to discuss the need for this vaccine with their health care provider.
PCV 15: Vaxneuvance™ (Pneumococcal 15-valent Conjugate Vaccine)
On July 16, 2021, Merck announced the FDA approval of Vaxneuvance™, a new vaccine for the prevention of invasive pneumococcal disease in adults 18 years and older caused by 15 serotypes.
PCV20: Prevnar 20™ (Pneumococcal 20-valent Conjugate Vaccine)
On June 8, 2021, Pfizer announced the FDA approval of the Prevnar 20™ vaccine for adults 18 years or older and noted that it is “the first approval of a conjugate vaccine that helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia, including seven responsible for 40% of pneumococcal disease cases and deaths in the U.S.”
PPSV23: Pneumovax23® (pneumococcal polysaccharide vaccine or PPSV23) is a Merck product. This vaccine was approved by the FDA in 1983 and helps protect against twenty-three types of pneumococcal bacteria.
The CDC recommends this vaccine for all adults 65 years or older, people 2 through 64 years old with certain medical conditions (i.e., diabetes, heart disease or COPD), and adults 19 through 64 years old who smoke cigarettes.
Respiratory Syncytial Virus (RSV) Vaccine
On May 3, 2023, the FDA announced they had approved Arexvy, the first RSV vaccine approved in the United States for the prevention of lower respiratory tract disease caused by RSV in people 60 years of age and older.
On June 29, 2023, the CDC endorsed the CDC Advisory Committee on Immunization Practices’ (ACIP) recommendations for use of the RSV vaccine in people ages 60 years or older. They noted that “Adults at the highest risk for severe RSV illness include older adults, adults with chronic heart or lung disease, adults with weakened immune systems, and adults living in nursing homes or long-term care facilities. CDC estimated that every year, RSV causes approximately 60,000-160,000 hospitalizations and 6,000-10,000 deaths among older adults.”
What Can I Do?
As a Healthcare Provider
Work with your patients to identify what vaccinations they have and have not received and utilize available resources on the CDC website for healthcare providers related to immunization schedules.
As a Healthcare Consumer
Keep your vaccination records up to date, use the CDC’s Adult Vaccine Assessment Tool to determine which vaccines are recommended for you, and share all this information with your healthcare provider so you make an informed decision on what immunizations you may need.
Resources
CDC webpages
- National Immunization Awareness Month: https://www.cdc.gov/vaccines/events/niam/
- Vaccine Information for Adults: https://www.cdc.gov/vaccines/adults/index.html
- Shingles Vaccination: https://www.cdc.gov/vaccines/vpd/shingles/public/shingrix/index.html
Merck July 16, 2021 Announcement: https://www.merck.com/news/merck-announces-u-s-fda-approval-of-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-adults-18-years-and-older-caused-by-15-serot/
Pfizer June 8, 2021 Announcement: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20tm-pfizers-pneumococcal-20-valent
FDA May 3, 2023 RSV Vaccine Announcement: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine?utm_medium=email&utm_source=govdelivery
June 29, 2023 CDC Announcement Recommending RSV Vaccine for Older Adults: https://www.cdc.gov/media/releases/2023/s0629-rsv.html
Beth Cobb
July is UV Safety Awareness Month. A related RealTime Medicare Data (RTMD) infographic in this week’s newsletter focuses on Medicare Fee-for-Service claims data related to the treatment costs of Melanoma.
Did You Know?
Anyone can get skin cancer, but people with certain characteristics are at greater risk—
A lighter natural skin color.
- Skin that burns, freckles, reddens easily, or becomes painful in the sun.
- Blue or green eyes.
- Blond or red hair.
- Certain types and many moles.
- A family history of skin cancer.
- A personal history of skin cancer.
- Older age.
Why It Matters?
Basal and Squamous Cell Carcinomas
According to the CDC (https://www.cdc.gov/cancer/skin/statistics/index.htm), skin cancer is the most common form of cancer in the United States. “An examination of Medical Expenditure Panel Survey data suggests that each year, about 6. 1 million adults are treated for basal cell and squamous cell carcinomas at a cost of about $8.9 billion.”
These numbers have increased exponentially from 2022 when the panel survey data suggested that each year about 4.3 million adults are treated for basal and squamous cell carcinomas at a cost of about $4.8 billion.
Melanoma
Following are recent National Cancer Institute cancer facts about melanoma:
- In 2020, there were an estimated 1,413,976 people living with melanoma of the skin in the U.S.
- Represents 5% of all new cancers in the U.S.
- Is more common in men than women.
- Is most frequently diagnosed among people ages 65-74 with a median age at diagnosis of 66.
- In 2023, it is estimated that there will be 97,610 new cases of melanoma of the skin and an estimated 7,990 people will die of this disease.
https://seer.cancer.gov/statfacts/html/melan.html
What Can I Do?
Be proactive in lowering your risk for melanoma and other skin cancers by following key sun safety tips from the FDA ( https://www.fda.gov/drugs/understanding-over-counter-medicines/sunscreen-how-help-protect-your-skin-sun):
- Limit time in the sun, especially between the hours of 10 a.m. and 4 p.m., when the sun’s rays are most intense,
- Wear clothing to cover skin exposed to the sun, such as long-sleeved shirts, pants, sunglasses, and broad-brimmed hats.
- Use broad spectrum sunscreens with SPF values of 15 or higher regularly and as directed.
- Reapply sunscreen at least every two hours, and more often if you are sweating or jumping in and out of the water.
Also, be mindful that certain medications can cause sensitivity to the sun, for example:
- Antibiotics (ciprofloxacin, doxycycline, levofloxacin, ofloxacin, tetracycline, trimethoprim),
- Antihistamines including Diphenhydramine (common brands include Benadryl and Nytol),
- Oral contraceptives and estrogens, and
- Non-steroidal anti-inflammatory drugs (ibuprofen, naproxen, celecoxib, piroxicam, ketoprofen).
You can read more about this on the FDA website (https://www.fda.gov/drugs/special-features/sun-and-your-medicine).
Beth Cobb
Did You Know?
According to the National Cancer Institute, bladder cancer:
- Is the fourth most commonly diagnosed malignancy in men in the United States,
- Occurs about four times higher in men than in women,
- Is diagnosed almost twice as often in White individuals as in Black individuals of either sex; and
- The incidence of bladder cancer increases with age.
Bladder Cancer Symptoms
Although symptoms can vary from person to person, the most common symptom is blood in the urine, called hematuria. Although hematuria is the most common presenting symptom, most people experiencing hematuria do not have bladder cancer. Other common symptoms include:
- Frequent urination,
- Pain or burning during urination,
- Feeling as if you need to urinate even if your bladder is not full, and
- Frequent urination during the night.
If the cancer has grown large or spread beyond the bladder, symptoms may include:
- Being unable to urinate
- Lower back pain on one side of the body
- Pain in the abdomen
- Bone pain or tenderness
- Unintended weight loss and loss of appetite
- Swelling in the feet, and
- Feeling tired.
April 3, 2023: FDA Grants Accelerated Approval for Patients
The FDA granted accelerated approval to enfortumab vedotin-ejfv (Padcev, Astellas Pharma) with pembrolizumab (Keytruda, Merck) for patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-containing chemotherapy. Note, this cancer primarily arises in the bladder.
In an April 3rd, Merck news release, Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories notes “This approval is a major milestone in the treatment of patients with locally advanced or metastatic urothelial carcinoma because it is the first approved combination of an immunotherapy and an antibody-drug conjugate for these patients…This expands the use of KEYTRUDA-based regimens to more patients with advanced urothelial carcinoma and demonstrates the value of collaboration in creating new combination approaches for patients in need of more options.”
Why it Matters?
There are risk factors related to developing bladder cancer, most common being tobacco use, especially smoking cigarettes. Examples of additional risk factors includes:
- Having a family history of bladder, cancer,
- Having certain changes in the genes that are linked to bladder cancer,
- Being exposed to paints, dyes, metals, or petroleum products in the workplace,
- Past treatment with radiation therapy to the pelvis or with certain anticancer drugs, such as cyclophosphamide or ifosfamide,
- Taking Aristolochia fangchi, a Chinese herb,
- Drinking water from a well that has high levels of arsenic,
- Drinking water that has been treated with chlorine,
- Having a history of bladder infections, and
- Using urinary catheters for a long time.
What Can I Do?
First, if you smoke, quit! If you think you may be at risk for bladder cancer and/or are experiencing symptoms common for bladder cancer, discuss this with your physician. Time matters. The earlier bladder cancer is identified, the better chance a person has of surviving five years after diagnosis. The current 5-year relative survival rate is 77.9%.
Resources:
National Cancer Institute Cancer Stat Facts: Bladder Cancer: https://seer.cancer.gov/statfacts/html/urinb.html
National Cancer Institute Bladder and Other Urothelial Cancers Screening (PDF®) Health Profession Version: https://www.cancer.gov/types/bladder/hp/bladder-screening-pdq
FDA April 3, 2023 News Release: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic
Merck April 3, 2023 New release: https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-in-combination-with-padcev-enfortumab-vedotin-ejfv-for-first-line-treatment-of-certain-patients-with-locally-advanced-or-metastatic/Beth Cobb
Did You Know?
The two most common types of esophageal cancer are squamous cell carcinoma and adenocarcinoma.
Squamous cell carcinoma is most often found in the upper and middle part of the esophagus but can occur anywhere along the esophagus. Studies have shown that the risk of squamous cell carcinoma of the esophagus increases in people who smoke or are heavy drinkers.
Adenocarcinoma usually forms in the lower part of the esophagus near the stomach. This type of esophageal cancer is strongly linked to gastroesophageal reflux disease (GERD), especially when severe symptoms occur daily. Obesity in combination with GERD may further increase your risk for adenocarcinoma of the esophagus.
In the last 20 years the rates of adenocarcinoma of the esophagus have increased in the United States and is now more common than squamous cell carcinoma of the esophagus.
Estimated New Cases and Deaths from Esophageal Cancer in the United States in 2023
- New Cases: 21,560
- Deaths: 16,120
Esophageal Cancer Risk Factors
- Tobacco Use
- Heavy alcohol use
- Barrett esophagus – Gastric reflux is the most common cause of Barrett esophagus.
- Men are about three times more likely than women to develop esophageal cancer.
- Older age
- White men develop esophageal cancer at higher rates than Black men in all age groups.
Signs and Symptoms of Esophageal Cancer
- Painful or difficult swallowing
- Weight loss,
- Pain behind the breastbone
- Hoarseness and cough
- Indigestion and heartburn
- A lump under the skin
Tests Used to Diagnose Esophageal Cancer
- Physical exam and health history,
- Chest x-ray,
- Esophagoscopy
- Biopsy
Why it Matters?
In most cases, esophageal cancer is a treatable but rarely curable disease. The five-year survival rate is 20.6%.
Patients have a better chance of recovery when esophageal cancer is found early. Only 18% of patients are diagnosed with esophageal cancer at the localized level. The five-year survival rate for this group of patients is 47.3%.
Signs and symptoms associated with esophageal cancer can also be present with other diseases. If you have any of the signs and symptoms mentioned in this article, discuss them with your doctor.
Resources:
PDQ® Adult Treatment Editorial Board. PDQ Esophageal Cancer Treatment (Adult). Bethesda, MD: National Cancer Institute. Updated 10/14/2022. Available at: >https://www.cancer.gov/types/esophageal/hp/esophageal-treatment-pdq. Accessed 3/31/2023. [PMID: 26389338]
PDQ® Screening and Prevention Editorial Board. PDQ Esophageal Cancer Prevention. Bethesda, MD: National Cancer Institute. Updated 07/30/2021 Available at: >https://www.cancer.gov/types/esophageal/patient/esophageal-prevention-pdq>. Accessed 3/31/2023. [PMID: 26389280]
Beth Cobb
February 22nd each year is National Heart Valve Disease Awareness Day. This day was started by the Alliance for Aging Research with a “goal…to increase recognition of the specific risks and symptoms of heart valve disease, improve detection and treatment, and ultimately save lives.”
Did You Know?
According to the Alliance for Aging Research:
- >As many as 11.6 million Americans are estimated to have heart valve disease (HVD),
- >Annually, around 25,000 people die from the disease, and
- Three out of four Americans know little to nothing about heart valve disease.
Causes of Heart Valvular Disease
- Rheumatic disease: An untreated infection from bacteria causing strep throat can cause scarring of the heart valve and it is the most common cause of valve disease worldwide. This is less common in the U.S. where strep infections are treated early with antibiotics.
- Endocarditis: When a severe infection in the blood causes an infection of the inner lining of the heart, the infection can settle on the heart valves and damage the leaflets. IV drug use can also lead to endocarditis and ultimately heart valve disease.
- Other types of heart disease i.e., heart failure, atherosclerosis, thoracic aortic aneurysm, high blood pressure or heart attack.
Risk Factors
- Older age can be a risk factor.
- A family history of coronary artery disease can raise your risk of developing HVD.
- Lifestyle habits that may put you at risk include a lack of physical activity, unhealthy eating patterns, smoking, and obesity.
- Other conditions that can raise your risk include high blood pressure, diabetes, and autoimmune disorders such as lupus.
- Radiation treatment for cancer can result in thickening or narrowing of heart valves.
- Sex, at all ages men are more likely than women to have certain heart valve conditions, such as aortic stenosis.
Symptoms in Adults
It is important to recognize that symptoms that occur in older patients may happen slowly, may be mistakenly thought to be normal signs of aging, or a patient may have no symptoms at all. When a patient does have symptoms, it can include:
- Fatigue, which is often the first symptom.
- Shortness of breath, especially on exertion
- Chest pain
- Dizziness, fainting when standing up, or a short-term loss of consciousness.
- Fever, which may signal an infection that can lead to endocarditis.
- Rapid weight gain, and
- Irregular heartbeat.
How Heart Valve Disease is Diagnosed
- Your doctor may hear a heart murmur during a physical examination and depending on the location, how it sounds and its rhythm, your doctor may be able to identify the valve and type of problem it is (regurgitation or stenosis).
- The above symptoms are like other conditions and your doctor can order an echocardiogram to diagnose a heart valve problem.
- How Heart Valve Disease is Treated
Medicine may treat symptoms and/or prevent the condition from worsening. Surgery or a minimally invasive structural heart procedure may ultimately be required to repair or fully replace a faulty heart valve.
Why it Matters?
Untreated HVD can lead to serious and even life-threatening complications for example:
- Arrhythmias,
- Blood clots,
- Blood stream infections,
- Expanding, bulging, or tearing of the aorta,
- Heart failure,
- Pulmonary hypertension (high blood pressure in the lungs),
- Stroke, or
- Cardiac Arrest.
What Can You Do?
Talk to your doctor about your risk during your routine examination and make healthy lifestyle changes (i.e., choose heart-healthy foods, maintain a healthy weight, manage stress, get regular physical activity, and if you smoke, quit).
References
- Alliance for Aging Research: https://www.agingresearch.org/aging-health/heart-valve-disease/
- The Alliance for Aging Research Heart Valve Awareness Day website at https://www.valvediseaseday.org/
- CDC Heart Valve Disease webpage: https://www.cdc.gov/heartdisease/valvular_disease.htm
- National Institutes of Health: National Heart, Lung, and Blood Institute: https://www.nhlbi.nih.gov/health/heart-valve-diseases
Beth Cobb
MMP has been sending out the Wednesday@One since 2012. Over the past decade, I have often shared with our readers my love of fall. Fall means the return of college football, front yards filled with inflatable pumpkins and ghosts, and this year I am seeing the addition of exceptionally large decorative black spiders crawling up the outside walls of homes and strings of glowing witch hats lighting front porches.
Even with pots of chili still to be cooked and caramel apples still to be consumed, it is never too early to prepare for the New Year. Along with the October 1st start of the CMS 2023 Inpatient Prospective Payment System (IPPS) Fiscal Year, this article highlights recent news to help you prepare for the coming year.
2023 Dollar Amount in Controversy Required for Administrative Law Judge (ALJ) Hearing or Federal District Court Review
The fifth level of appeal for Medicare Fee-for-Service appeals is an ALJ hearing or Federal District Court review. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA), requires an annual reevaluation of the dollar amount in controversy (AIC) required to advance to this level of appeal.
On September 30, 2022, the annual adjustment that will be effective on January 1, 2023 was published in the Federal Register (link). The calendar year (CY) 2023 AIC threshold amounts are:
- ALJ hearing requests filed on or after January 1, 2023 remains the same as CY 2022 at $180.
- Federal District Court requests filed on or after January 1, 2023 will increase from the CY 2022 amount of $1,760 to $1,850.
You can learn more about the appeal process in the CMS MLN Booklet Medicare Parts A & B Appeals Process (link).
Inflation Reduction Act
President Biden signed the Inflation Reduction Act (IRA) into law on August 16, 2022. On October 5th, CMS released a Fact Sheet (link) where CMS notes that “this law means millions of Americans across all 50 states, the United States territories, and the District of Columbia will save money from meaningful benefits.” Insulin cost sharing is one of the benefits that will start in 2023 and includes:
- Starting January 1, 2023, people enrolled in a Medicare prescription drug plan will not pay more than $35 for a month’s supply of each insulin that they take and is covered by their Medicare prescription drug plan and dispensed at a pharmacy or through a mail-order pharmacy. Also, Part D deductibles will not apply to the covered insulin product.
- Starting July 1, 2023, people with traditional Medicare who take insulin through a traditional pump will not pay more than $35 for a month’s supply of insulin, and the deductible will not apply to the insulin. This will apply to people using pumps covered through the durable medical equipment benefit under Part B.
COVID-19 PHE Extended
The Secretary of Health and Human Services, Xavier Becerra, renewed the COVID-19 public health emergency this past Thursday, October 13th (link). As a reminder, PHE declarations last for the duration of the emergency or 90 days and may be extended by the Secretary. Ninety days from October 13th will be January 11th, 2023. Specific to the COVID-19 PHE, HHS has indicated that they will provide a 60-day notice prior to the termination of the COVID-19 PHE. Sixty days prior to January 11, 2023 is Saturday, November 12th, 2022.
Social Security Benefits in 2023
In an October 13th Press Release (link), the Social Security Administration announced that “approximately 70 million Americans will see a 8.7% increase in their Social Security benefits and Supplemental Security Income (SSI) payments in 2023. On average, Social Security benefits will increase by more than $140 per month starting in January.”
Calendar Year 2023 Medicare Deductible, Coinsurance & Payment Rates
Since writing about the updated Medicare deductible, coinsurance and payment rates in last week’s newsletter (link), CMS has published MLN Matters article MM12903 (link) which includes background information regarding a Medicare beneficiary’s “spell of illness” and Medicare coverage in a skilled nursing facility (SNF) as well as the 2023 payment rate changes.
As we wait for the release of the CY 2023 Outpatient Prospective Payment System (OPPS) Final Rule, the 2022 CERT Report, and the possible notification of the end of the COVID-19 PHE, I wish all our readers a happy fall y’all.
Did You Know?
- An estimated 12.1 million people will have A-Fib in 2030,
- In 2019, A-fib was mentioned on 183,321 death certificates and was the underlying cause of death in 26,535 of those deaths,
- People of European descent are more likely to have A-fib than African Americans, and
- Because the number of A-fib cases increases with age and women generally live longer than men, more women than men experience A-fib.
Why it Matters?
- More than 454,000 hospitalizations with A-fib as the primary diagnosis happen each year in the United States,
- A-fib increases a person’s risk of stroke. In fact, A-fib causes 1 in 7 strokes and strokes caused by A-fib tend to be more severe than strokes with other underlying causes, and
- The death rate from A-fib as the primary or a contributing cause of death has been rising for more than two decades.
What Can I Do?
Know the risk factors for A-fib
- Advancing age,
- Family member with a history of A-fib increases your chances of having A-fib,
- High blood pressure,
- Obesity,
- European ancestry,
- Diabetes,
- Heart failure,
- Ischemic heart disease,
- Hyperthyroidism,
- Chronic Kidney Disease,
- Moderate to heavy alcohol use,
- Smoking,
- Enlargement of the chambers on the left side of the heart,
- A-fib is the most common complication after heart surgery,
Know the symptoms of A-fib
- Irregular heartbeat,
- Heart palpitations (rapid, fluttering, or pounding),
- Lightheadedness,
- Extreme fatigue,
- Shortness of breath, and
- Chest pain.
Note, it is possible to have no symptoms, or in my mom’s experience, she thought was having panic attacks when on further study by her physician, she was experiencing episodes of A-fib.
Know Common “Triggers” That May Cause an Episode of A-fib
- Caffeine and energy drinks. The American Heart Association notes that “although normal amounts of coffee shouldn’t trigger Afib, further study may be warranted for energy drinks and excessive caffeine intake.”
- Excessive alcohol,
- Stress or anxiety, and
- Poor sleep and/or sleep apnea.
Know the Treatment Options
- Medicines to control your heart’s rhythm and rate,
- Non-surgical procedures (i.e., electrical cardioversion and radiofrequency ablation), and
- Surgical procedures (i.e., pacemaker, left atrial appendage closure implant (Watchman™) for non-valvular A-fib).
While other conditions can cause similar symptoms, if you experience any symptoms of A-fib, contact your doctor. If you are diagnosed with A-fib there is good news. According to the American Heart Association, “people can live long healthy and active lives with AFib. Controlling your risk factors for heart disease and stroke and knowing what can possibly trigger your AFib will help improve your long-term management of AFib.”
Resources
- CDC Atrial Fibrillation (A-fib): https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- American Heart Association: https://www.heart.org/en/health-topics/atrial-fibrillation/who-is-at-risk-for-atrial-fibrillation-af-or-afib
Beth Cobb
This past weekend my brother and I had the daunting task of downsizing my mom’s living space from an Assisted Living Facility apartment to a long-term care room. While a tough move for my mom, we did find a few hidden treasures and memories. One such memory was finding pictures from a 1976 vacation taken by my grandmother aboard a cruise ship that was part of the 1970s TSS Mardi Gras, The Golden Fleet Carnival Cruise Line. In addition to finding the pictures, there was a packet of daily activities and a map of the different levels of the ship.
In keeping with the cruise ship treasures that we found, this week we celebrate the 12th annual Clinical Documentation Integrity (CDI) Week with the theme Under the Sea-DI. A CDI Week Fact Sheet (link) published by the Association of Clinical Documentation Integrity Specialists (ACDIS), indicates that “CDI specialist review patient medical records and assess whether all conditions and treatments are documented. This documentation helps paint an accurate picture of the severity of the patient’s illness and the extent of the care required. When the documentation is unclear or deficient, CDI specialists prompt (also known as “query”) physicians to provide clarification. CDI specialists serve as the bridge between health information management (HIM) and clinical staff. They must comply with Medicare and/or private payer rules and regulations.”
Just as it takes the entire crew to make a cruise ship run smoothly, it takes the CDI team coordinating with doctors, other departments participating in the care of a patient (i.e., physical therapy, dietician, pharmacy), and coding professionals to find all the hidden treasure in a patient’s medical record.
MMP would like to wish all the hard-working CDI Professionals that we have the privilege to work with a happy CDI week. To help you prepare for the new CMS fiscal year, while celebrating this week, following are links to key treasure for a successful start to the CMS FY 2023.
FY 2023 IPPS Final Rule Home Page (link)
On this webpage you will find a links to:
- The FY 2023 IPPS Final Rule,
- FY 2023 Final Rule Tables
- Table 5: MS-DRGs, Relative Weighting Factors, Geometric and Arithmetic Mean Lengths of Stay, and Post-Acute Transfer designated MS-DRGs
- Table 6: New Diagnosis Codes,
- Table 6B: New Procedure Codes
- Table 6I: Complete MCC List,
- Table 6I.1: Additions to the MCC List,
- Table 6I.2: Deletions to the MCC List,
- Table 6J: Complete CC list,
- Table 6J.1: Additions to the CC list,
- Table 6J.2: Deletions to the CC list
- FY 2023 MAC Implementation Files
- MAC Implementation File 7: FY 2023 MS-DRGs Subject to the Replaced Devices Policy,
- MAC Implementation File 8: FY 2023 New Technology Add-on Payment
2023 ICD-10-CM Files (link)
Downloads available on this webpage includes:
- 2023 POA Exempt Codes,
- 2023 Conversion Table,
- 2023 Code Description in Tabular Order,
- 2023 Addendum,
- 2023 Code Tables, Tabular and Index, and
- FY 2023 ICD-10-CM Coding Guidelines.
The ICD-10-Files are also available on the CDC’s Comprehensive Listing ICD-10-CM Files webpage (link).
2023 ICD-10-PCS Files (link)
Downloads available on this webpage includes:
- 2023 ICD-10-PCS Order File,
- 2023 Official ICD-10-PCS Coding Guidelines,
- 2023 Version Update Summary,
- 2023 ICD-10-PCS Codes File,
- 2023 ICD-10-PCS Conversion table, 2023 ICD-10-PCS Code Tables and Index, and
- 2023 ICD-10-PCS Addendum.
MS-DRG Definitions Manual and Software
The ICD-10 MS-DRG Version 40 (V40) Grouper Software, ICD-10 MS-DRG Definitions Manual, and the Definitions of Medicare Code Edits V 40 files are publicly available on the CMS MS-DRG Classifications and Software webpage (link).
Again, happy CDI week from our team to yours.
Anita Meyers
No Results Found!
Yes! Help me improve my Medicare FFS business.
Please, no soliciting.
We are an environmentally conscious company, dedicated to living “green” both at work and as individuals.
© Copyright 2020 Medical Management Plus, Inc.
