Knowledge Base Article
PSMA PET Imaging for Prostate Cancer

NOTE: All in-article links open in a new tab.
September is Prostate Cancer Awareness Month. Prostate cancer is the second leading cause of male cancer-related death in the U.S.[1] According to the American Cancer Society, it is estimated that in 2023 there will be 288,300 new cases of prostate cancer and 34,700 prostate-cancer related deaths in the U.S.[2]
Historically, there have been limited options in managing patients with advanced prostate cancer. However, in the last several years, we have seen remarkable progress in the development of new diagnostic and therapeutic tools. One of these, PSMA PET imaging for prostate cancer, is a particularly exciting development and is the focus of this article. Medical oncologist Michael Morris from Memorial Sloan Kettering Cancer Center calls this new imaging technology “the biggest advance in prostate cancer detection since the PSA test was developed in the 1980s.”[3]
PSMA PET Imaging: A New Diagnostic Tool
Prostate-Specific Membrane Antigen, or PSMA, is a protein that is present at a higher level in prostate cancer cells, and in addition, is often found on the surface of prostate cells.[4] These characteristics of PSMA make it a good target for imaging prostate cancer that might have escaped from the prostate and traveled to other parts of the body. PSMA should not be confused with Prostate-Specific Antigen, or PSA, which is a protein produced by the prostate.[5] The PSA test measures the level of PSA in the blood. An elevated PSA in the blood can be an indication of prostate cancer, although it can be due to other factors.
Imaging for advanced prostate cancer has been problematic for many years, with men often having to undergo a conventional CT scan and a bone scan to see if there is evidence of metastatic disease. However, according to the National Cancer Institute, both of these conventional imaging technologies have limitations since “neither is particularly good at finding individual prostate cancer cells, and thus can miss very small tumors.”[6] PSMA PET imaging promises to improve the sensitivity of detecting prostate cancer metastases compared to conventional imaging approaches, and thereby better inform the treatment and management of patients with advanced disease.[7]
Clinical trials have shown some promising results for this new imaging technology. For example:
- In the CONDOR trial, a total of 208 men were enrolled in the study. The men had a rising PSA after surgery or radiotherapy. The study evaluated the radiotracer 18F-DCFPyL and its ability to detect prostate cancer in these men when performing a PET/CT. The trial demonstrated that the radiotracer correctly localized disease in approximately 85% of men with prostate cancer biochemical recurrence, all of whom had uninformative conventional imaging.[8]
- In another trial, 276 prostate cancer patients were enrolled to evaluate the clinical impact of 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography on the planned management of prostate cancer patients with biochemical recurrence after surgery.[9] It was found that the use of this imaging technology allowed clinicians to radically change the intended treatment approach before imaging evaluation, in roughly two out three individuals.
FDA Approvals
During the last several years, the FDA has approved several radioactive tracers for use in PSMA PET imaging. For example:
- On December 1, 2020, the FDA approved the radioactive tracer Gallium (Ga) 68 PSMA-11 for use in PET imaging of patients with suspected prostate cancer metastasis who are potentially curable by surgery or radiation therapy.[10] The tracer can also be used for patients with suspected prostate cancer recurrence based on elevated serum PSA levels.
- On May 26, 2021, the FDA approved a second PSMA-targeted PET imaging drug, Pylarify (piflufolastat F 18), for the same prostate cancer imaging indications as Ga 68 PSMA-11.[11] The FDA noted that with this approval, certain men with prostate cancer will have greater access to PSMA-targeted PET imaging that can aid health care providers in assessing prostate cancer.
Additional FDA approvals have followed for Illuccix (gallium Ga 68 gozetotide) (12/17/2021)[12], Locametz (gallium Ga 68 gozetotide) (3/23/2022)[13], and Posluma (flotufolastat F 18) (5/25/2023)[14].
Once a PSMA-targeted radioactive tracer is injected into the patient, the tracer travels throughout the body and attaches to PSMA; the cells thus flagged will then “light up” when a PET scan is performed.[15]
PSMA Tracers by the Numbers
To measure the growth in utilization of this new imaging technology, RealTime Medicare Data constructed a Tableau visualization using its nationwide Medicare Fee-for-Service (FFS) paid claims database. Here is some key trending information from that visualization:
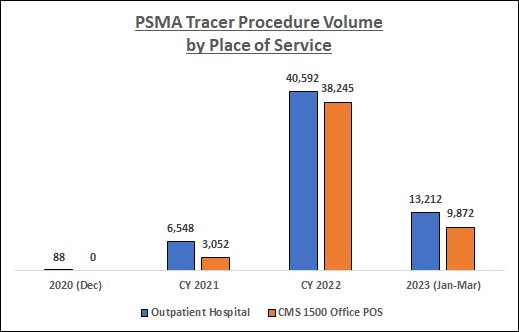
Data Source: RealTime Medicare Data, LLC. Time period: 12/1/2020-3/31/2023. Geography: all 50 states and D.C. CMS 1500 Office POS and Outpatient Hospital Medicare Fee-for-Service. The following HCPCS Codes were included in the data queries: A9593-GALLIUM GA-68 PSMA-11 DIAGNOSTIC UCSF 1 MCI, A9594-GALLIUM GA-68 PSMA-11 DIAGNOSTIC UCLA 1 MCI, A9595-PIFLUFOLASTAT F-18 DIAGNOSTIC 1 MCI, A9596-GALLIUM GA-68 GOZETOTIDE DIAG ILLUCCIX 1 MCI, A9597-POSITRON EMISSION TOMOGRAPHY RP DX TUMOR ID NOC, and A9800-GALLIUM GA-68 GOZETOTIDE DIAGNOSTIC 1 MCI. PDx's not related to prostate cancer, and CPT Modifier 26 (relating to professional fees), were filtered from the data.
As the above chart indicates, PSMA tracer utilization among the Medicare FFS population has increased substantially over the study period, especially during CY 2022. In addition, there has been a shift in the place of service where these procedures are being performed; indeed, by 2022 procedure volume in the Office setting was fast approaching that in the Outpatient Hospital setting.
From Imaging to Targeted Therapy
In addition to being a target for prostate cancer imaging, can PSMA be a target for prostate cancer therapy? In 2022, the FDA weighed into this question with these exciting developments:
- On March 23, 2022, the FDA approved Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for the treatment of adult patients with PSMA-positive metastatic castration-resistant prostate cancer who have been treated with androgen receptor pathway inhibition and taxane-based chemotherapy.[16]
- On the same day, the FDA approved the radioactive tracer Locametz (gallium Ga 68 gozetotide) for positron emission tomography (PET) of PSMA-positive lesions, including the selection of patients with metastatic prostate cancer for whom lutetium Lu 177 vipivotide tetraxetan PSMA-directed therapy is indicated.[17] The FDA noted that Locametz is the first radioactive tracer approved for patient selection in the use of a radioligand therapeutic agent.
Pluvicto acts by binding to PSMA; a radioactive particle then kills the cancer cells.[18] In a clinical trial leading to the FDA approval, the trial demonstrated a statistically significant improvement in the primary endpoints of overall survival and radiographic progression-free survival.[19]
Questions remain as to who might be able to receive the new therapy drug beyond those who have already been treated with chemotherapy, whether it will benefit patients during earlier stages of prostate cancer, and whether its effectiveness will be improved if combined with other therapies.[20]
Disclaimer: This article does not provide medical advice. It is intended for general informational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read in this article.
[1] American Cancer Society. Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html. Accessed 9/10/2023.
[2] Ibid.
[3] Memorial Sloan Kettering Cancer Center. New Technology that Pinpoints Prostate Cancer Will Transform Care. March 26, 2021. https://www.mskcc.org/news/new-technology-pinpoints-prostate-cancer-will-transform-care. Accessed 9/10/2023.
[4] Stallard J. Memorial Sloan Kettering Cancer Center. PSMA: A New Target for Prostate Cancer Treatment. Nov. 15, 2017. https://www.mskcc.org/news/psma-new-target-prostate-cancer-treatment. Accessed 9/10/2023.
[5] National Cancer Institute. Prostate-Specific Antigen (PSA) Test. https://www.cancer.gov/types/prostate/psa-fact-sheet. Accessed 9/10/2023.
[6] National Cancer Institute. PSMA PET-CT Accurately Detects Prostate Cancer Spread, Trial Shows. May 11, 2020 as updated. https://www.cancer.gov/news-events/cancer-currents-blog/2020/prostate-cancer-psma-pet-ct-metastasis. Accessed 9/10/2023.
[7] UCSF Department of Radiology & Biomedical Imaging. Prostate Specific Membrane Antigen (PSMA) PET Imaging for Prostate Cancer. https://radiology.ucsf.edu/psma-pet-scan-for-prostate-cancer. Accessed 9/10/2023.
[8] Morris J et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res (2021) 27 (13): 3674–3682. https://doi.org/10.1158/1078-0432.CCR-20-4573. Accessed 9/10/2023.
[9] Bianchi L et al. How does 68 Ga-prostate-specific membrane antigen positron emission tomography/computed tomography impact the management of patients with prostate cancer recurrence after surgery?
Int J Urol. 2019 Aug;26(8):804-811. doi: 10.1111/iju.14012. Epub 2019 May 13. PMID: 31083784. https://pubmed.ncbi.nlm.nih.gov/31083784/. Accessed 9/10/2023.
[10] U.S. Food & Drug Administration. FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Dec. 1, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer. Accessed 9/10/2023.
[11] U.S. Food & Drug Administration. FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. May 27, 2021. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer. Accessed 9/10/2023.
[12] U.S. Food & Drug Administration. CY 2021 CDER Drug and Biologic Calendar Year Approvals As of December 31, 2021. https://www.fda.gov/media/158149/download. Accessed 9/10/2023.
[13] U.S. Food & Drug Administration. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. March 23, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed 9/10/2023.
[14] U.S. Food & Drug Administration. Novel Drug Approvals for 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023. Accessed 9/10/2023.
[15] Rath L. WebMD. PSMA and Detecting Recurring Prostate Cancer. April 18, 2023. https://www.webmd.com/prostate-cancer/prostate-cancer-psma-detect. Accessed 9/10/2023.
[16] U.S. Food & Drug Administration. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. March 23, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed 9/10/2023.
[17] Ibid.
[18] Schmidt C. New treatment approved for late-stage prostate cancer. Harvard Health Publishing. Harvard Medical School. April 7, 2022. https://www.health.harvard.edu/blog/new-treatment-approved-for-late-stage-prostate-cancer-202204072722. Accessed 9/10/2023.
[19] U.S. Food & Drug Administration. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. March 23, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed 9/10/2023.
[20] Schmidt C. Ibid.
This material was compiled to share information. MMP, Inc. is not offering legal advice. Every reasonable effort has been taken to ensure the information is accurate and useful.
Yes! Help me improve my Medicare FFS business.
Please, no soliciting.
We are an environmentally conscious company, dedicated to living “green” both at work and as individuals.
© Copyright 2020 Medical Management Plus, Inc.



